Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in Google Play or Apple App Store. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on 01753 627777.
A link to the prescribing information can be found in the header.
Dysport® (Clostridium botulinum type A toxin-haemagglutinin complex) is indicated in adults for symptomatic treatment of spasmodic torticollis
Please click here to see the full therapeutic indications.

CLIMB Online
Cervical Dystonia

Dysport® Injection Handbook
A reference guide to the administration of Dysport for the treatment of cervical dystonia in adults, including the identification of muscles using ultrasound, and the recommended injection technique per muscle.
DYS-UK-008778
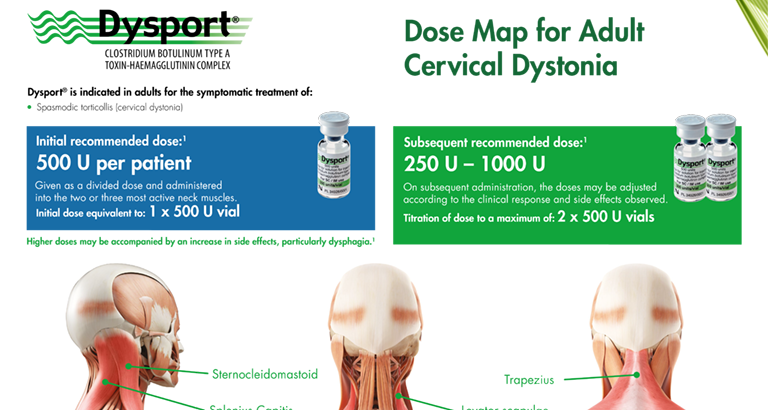
Dysport® Dose Map for Cervical Dystonia
A reference guide to the dosing of Dysport® for the symptomatic treatment of cervical dystonia.
DYS-UK-008781

Cervical Dystonia Disease Background – eLearning Module
20 minutes
An overview of clinical characteristics of cervical dystonia (CD), the sub-types of CD, and the impact CD can have on patients
DYS-UK-008759

Dysport® Clinical Studies in Cervical Dystonia – eLearning Module
20 minutes
An overview of the phase 3 clinical studies of Dysport® in cervical dystonia
DYS-UK-008942




















